Identification of key epithelial-mesenchymal interactions in cancer
Identification of key epithelial-mesenchymal interactions in cancer
Despite advances in early detection and treatment of oral cancer, mortality from this disease remains high because current therapies are limited by the emergence of secondary field tumors and of therapy resistant recurrences. Treatment strategies used nowadays against oral squamous cell carcinoma (OSCC) are based on the classical view that a tumor is composed of a homogenous population of multiplying cells that harbor certain genetic changes responsible for their invasive and metastasizing behavior. This concept overlooks (1) the fact that tumors contain heterogeneous cell populations with different self-renewing and regenerative potential, and (2) the role that local microenvironment (e.g. cancer-associated fibroblasts) play on behavior of tumor cells. Development of novel and more efficient oral cancer treatment strategies that take into consideration these factors is thus required, and for this purpose more basic research for understanding the biology of oral cancer and carcinomas in general is needed. This research project is concentrated on two main areas: (1) understanding how the collaborative interactions between human transformed epithelial cells and stromal fibroblasts result in their ability to invade and metastasize; and (2) the molecular mechanisms of self-renewal in carcinomas, how they are affected by tumor microenvironment and how they can be targeted. Recently, we have identified by gene microarray four TGFb1-related molecules (PMEPA1, BGN, CADPS and ITGA11) that are up-regulated in CAF and associated with increased oral cancer cell invasion and poor patient survival (Costea et al, Cancer Res, 2013). The task now is to reveal the mechanisms by which they alter cancer cell invasion and metastasis and determine how they can be targeted.
Relevance
Understanding the role that cancer associated fibroblasts (CAF), the most abundant cell type of tumor stroma, play on oral cancer cell survival and expansion will (1) increase the knowledge on the specific factors that promote cancer invasion, and (2) may yield additional targets for novel therapeutic strategies in which both transformed cells and activated stroma are co-targeted.
Identification of the factors responsible for the phenotypic equilibrium and switch between the various subpopulations of cells in normal and neoplastic human oral mucosa
 Cell-phenotypic dynamics are of particular importance in tumor progression and several phenotypic cancer cells frequently co-exist in individual tumors. Cancer cells with distinct phenotypes often show important differences in term of their functional properties. Increasing evidence has identified the existence of both epithelial-mesenchymal-transition (EMT) and epithelial cancer stem cell (CSC) phenotypes in vitro systems, and our previous work has demonstrated that these two distinct phonotypic cell populations can coexist and switch in both directions (Biddle etal, Cancer Res, 2011). However, evidence for heterogeneous CSCs phenotypes in human tumors is relatively sparse. Furthermore, pilot work in our collaborative laboratory has also demonstrated the coexistence of epithelial and EMT cancer stem cell phenotypes in fresh tumor specimens from OSCC patients. However, the mechanism underlying of this relation in human tumors has barely been investigated. Most recent studies are showing that cancer cell populations were able to retain phenotypic equilibrium over extended periods of time, providing that it is possible through one or more interconversions to transition between any heterogeneous phenotypes. In addition, the plasticity of cancer stem cells refers to their ability to reconstitute the cellular heterogeneity typical of the original tumor, although the classic definition of cell plasticity taken from stem cell biology implies the ability of stem cells to differentiate into various cell lineages. The term plasticity is also currently used to define the ability of a given cell type to reciprocally dedifferentiate, redifferentiate, and/or transdifferentiate in response to specific stimulation. In this sense, it will be of significant interest to determine how CSCs acquire plasticity and maintain their phenotypic equilibrium and switching under specific modulations since these modulations of the underlying mechanisms could lead to novel therapies for regenerative medicine. Our working hypothesis for this project is that there is a phenotypic equilibrium between different subpopulations of cells in both normal and neoplastic oral mucosa; and the maintenance of this equilibrium and the dynamic of the switch between various sub-populations of cells can be altered by several factors, such as exogenous factors derived from surrounding stroma. The aim is to understand the dynamic/kinetics of the switch between various sub-populations of cells and how this equilibrium is maintained at the molecular level.
Cell-phenotypic dynamics are of particular importance in tumor progression and several phenotypic cancer cells frequently co-exist in individual tumors. Cancer cells with distinct phenotypes often show important differences in term of their functional properties. Increasing evidence has identified the existence of both epithelial-mesenchymal-transition (EMT) and epithelial cancer stem cell (CSC) phenotypes in vitro systems, and our previous work has demonstrated that these two distinct phonotypic cell populations can coexist and switch in both directions (Biddle etal, Cancer Res, 2011). However, evidence for heterogeneous CSCs phenotypes in human tumors is relatively sparse. Furthermore, pilot work in our collaborative laboratory has also demonstrated the coexistence of epithelial and EMT cancer stem cell phenotypes in fresh tumor specimens from OSCC patients. However, the mechanism underlying of this relation in human tumors has barely been investigated. Most recent studies are showing that cancer cell populations were able to retain phenotypic equilibrium over extended periods of time, providing that it is possible through one or more interconversions to transition between any heterogeneous phenotypes. In addition, the plasticity of cancer stem cells refers to their ability to reconstitute the cellular heterogeneity typical of the original tumor, although the classic definition of cell plasticity taken from stem cell biology implies the ability of stem cells to differentiate into various cell lineages. The term plasticity is also currently used to define the ability of a given cell type to reciprocally dedifferentiate, redifferentiate, and/or transdifferentiate in response to specific stimulation. In this sense, it will be of significant interest to determine how CSCs acquire plasticity and maintain their phenotypic equilibrium and switching under specific modulations since these modulations of the underlying mechanisms could lead to novel therapies for regenerative medicine. Our working hypothesis for this project is that there is a phenotypic equilibrium between different subpopulations of cells in both normal and neoplastic oral mucosa; and the maintenance of this equilibrium and the dynamic of the switch between various sub-populations of cells can be altered by several factors, such as exogenous factors derived from surrounding stroma. The aim is to understand the dynamic/kinetics of the switch between various sub-populations of cells and how this equilibrium is maintained at the molecular level.
Relevance
Understanding how cancer cell states coexist and dynamically evolve within tumors is of fundamental interest and could facilitate the development of more effective therapies, since that anticancer therapies preferentially kill specific cancer cells, treatment can result in selective changes in phenotypic proportions within tumors.
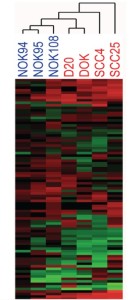 Development and validation of a molecular diagnostic tool for early diagnostic and personalized treatment of oral cancer
Development and validation of a molecular diagnostic tool for early diagnostic and personalized treatment of oral cancer
Oral cancer is the 15th most common cancer worldwide (source: GLOBOCAN 2008) and a major health problem in South-Central India (the 2rd most common), with increasing incidence due to poor patient accessibility to healthcare and the lack of effective diagnostic tests for early cancer detection (Gupta et al, Int Dent J., 2013). We have developed an easily accessible and quantitative Malignancy Index Diagnostic System (q-MIDS) using a quantitative PCR to detect early signs of oral cancer based on molecular signature of 16 biomarkers implicated in the regulation of the cell cycle, genomic stability, chromatin maintenance, and stem cell regulation (Teh et al, Int J Cancer, 2012). qMIDS provided objective, quantitative results rapidly on very small biopsies (1-2mm3) rendering biopsy collection a simple procedure. It has been successfully validated using tissue biopsies from patients in Norway and UK, accurately discriminating between normal, pre-cancer and neoplastic oral tissue with high efficiency (>90%) and low false positive rate (<5%). We have also identified additional prognostic biomarkers in the tumor stroma (20 TGFβ related molecules) and validated them on patient tissues from Norway and UK (Costea et al, Cancer Res., 2013). We are now in the process of including them in the q-MIDS system and validating it on Norwegian, UK, Indian and Nepalese patients, both at q-PCR and immuno-histo-chemistry (IHC) level since the IHC-MIDS is more feasible in the rural settings of South-Central India. Early diagnosis is the most effective determinant of cancer disease control and avoids morbidity from complex and costly long term treatment. The key objective of this proposal is to establish and execute a multicenter study in Western Europe and South-Central India to validate and advance q-MIDS / IHC-MIDS towards commercialization and integration into the Healthcare system for early oral cancer diagnosis.
Relevance
The accessibility of IHC-MIDS to rural populations and its speed and sensitivity for early cancer detection may potentially revolutionize oral cancer diagnosis and improve patient survival.
 Investigation of cell-bacterial interactions and effects on normal and neoplastic human oral mucosa
Investigation of cell-bacterial interactions and effects on normal and neoplastic human oral mucosa
The epithelial cells of junctional epithelium and sulcular epithelium are constantly challenged by the bacteria of the subgingival biofilm. Normally, the exfoliation rate of the epithelial cells and the immune response keep a balance between the bacterial aggression and the integrity of the periodontium. Intracellular oral bacteria may escape the immune response, may constitute a reservoir for recolonization of dental sites after periodontal treatment and may possibly spread towards deeper layers or even into blood stream. F. nucleatum, a key member of the subgingival biofilm, possesses the FadA protein on the outer membrane, which functions as an adhesin, allowing the microorganism to bind to and enter different host cell types. F. nucleatum may also enhance invasion of periopathogen P. gingivalis in gingival epithelial cells and may also transport intracellularly other non-invading bacteria. We have shown that this bacterium is able to enter also oral fibroblasts, the main cell type in the conjunctive tissue, but also advance within superficial epithelial layers of a multilayer model of gingival mucosa. Important steps may be achieved in understanding the mechanisms through which periodontal bacteria undermine the periodontium by using in vitro investigating tools that mimic as close as possible the situation encountered in vivo. In this project, we want to investigate further the interactions between the opportunistic commensal F. nucleatum and the periopathogen P. gingivalis and the host tissues. To gain a better understanding of how oral bacteria contribute to the periodontium destruction, we want to challenge a multilayer model of junctional epithelium with oral bacteria in mono or multispecies biofilm. Furthermore, we want to identify the binding partners of fusobacterial adhesins on the suface of oral epithelial and fibroblast cells.
Relevance
Understanding the fine mechanisms involved in oral bacteria adhesion to and invasion into host tissues might bring new insight on how periodontal disease and other systemic conditions may be prevented.
Investigation of nanoparticle penetration and effects on normal and neoplastic human oral mucosa
For as long as humans have existed, humans have been exposed to nanoparticles (NPs). These NPs can originate from natural sources, e.g. volcanic ashes, smoke, dust. The recent progress of nanotechnology, where matter is manipulated at the atomic level, has given rise to a high variety of manmade, engineered nanomaterials and particles whose properties are difficult to predict. Data on how these particles interact with humans and the environment is insufficient. Very little is known about the ability of NPs to cross the oral mucosal barrier. The oral mucosa can be exposed to NPs in foods, tooth paste, dental materials, or released from dental fillings especially during polishing. The aim of this study is to investigate the NPs’ ability to cross the normal and neoplastic oral mucosa and its effects on the exposed tissues.
Relevance
Such information can be used both for theranostics and for the evaluation of NPs’ putative toxic effects.